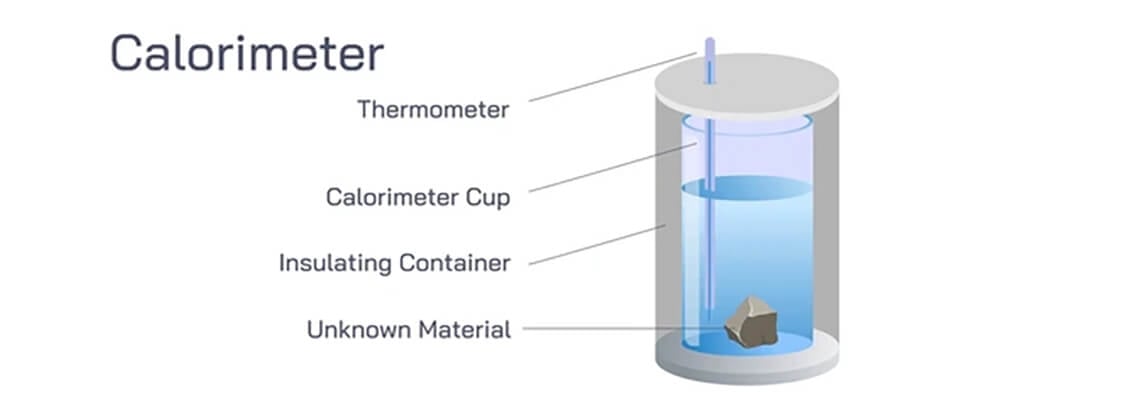
If you’re asking ‘what does a calorimeter measure,’ you’re referring to a device that gauges the amount of heat involved in chemical reactions or physical processes. This measurement is critical for determining whether these reactions release or consume energy. Our discussion will lead you through the operational principles of calorimeters, their various types, and their application in various scientific and industrial contexts.
Key Takeaways
- Calorimetry measures heat related to chemical reactions or changes in a substance’s heat capacity, classifying reactions as exothermic or endothermic and informing various scientific and industrial fields. In this blog, we will discover how and what does a calorimeter measure.
- Different types of calorimeters such as bomb, adiabatic, and differential scanning calorimeters help measure heat changes in a vast range of environments, encompassing applications from educational settings to industrial processes.
- While calorimetry is essential for understanding thermodynamics and facilitating safe and optimized processes, it faces challenges such as heat loss, the need for regular calibration, and sensor limitations that can affect measurement accuracy.
What Does a Calorimeter Measure: The Fundamentals of Calorimetry
Imagine quantifying the heat involved in a process, whether in a high school lab or a complex industrial setting. That’s the world calorimetry introduces us to. A calorimeter primarily measures the heat released or absorbed in reactions and when characterizing a material’s heat capacity. This function of calorimetry helps us classify reactions as exothermic, which releases heat, or endothermic, which absorbs heat.
The key to measuring the precise heat involved in a reaction lies in isolating the reaction within a calorimeter. This isolation is a barrier against heat loss to the surrounding environment, which guarantees accurate measurements. Consequently, the calorimeter measures the heat directly involved in the reaction, eliminating any external influences.
Calorimetry aids in comprehending the unique characteristics of different materials. It provides an accurate measure of a material’s heat capacity, a piece of information that’s indispensable across a range of fields, from construction to culinary arts.
Heat Capacity and Specific Heat
But what exactly do we mean by heat capacity when we discover what does a calorimeter measure? Raising the temperature of an object by one degree Celsius or Kelvin requires a specific amount of heat. The mass and type of material influence this measurement, quantified in joules per kelvin (J/K).
Now, let’s consider an interesting variant of heat capacity – specific heat capacity. This measures the heat required to raise the temperature of one unit of mass of a substance by one degree Celsius or Kelvin. As an intensive property, specific heat capacity does not depend on the mass of the substance and is expressed in joules per kelvin per kilogram (J/K/kg). The specific heat capacity of different materials accounts for their varying rates of heating and cooling. For instance, water has a higher resistance to temperature changes compared to sand.
To calculate thermal energy with a temperature rise for heat capacity, we use the equation Q = CΔT, where Q represents the amount of heat, C is the heat capacity, and ΔT indicates the temperature change.
For specific heat capacity, the formula Q = mcΔT is used, with Q as the heat amount, m as the mass, c as the specific heat capacity, and ΔT the temperature change.
Heat Flow and Temperature Difference
As we delve deeper into the complexities of calorimetry and what a calorimeter measures, we come across the concept of heat flow. Calorimeters measure this by monitoring temperature changes within the system resulting from a chemical or physical process. To accurately measure heat transfer, the system and its surroundings are clearly defined. Heat exchange between them is quantified through temperature changes.
One of the fundamental principles that calorimetry relies on is the principle of energy conservation. This principle ensures that ideally there is no heat loss to the calorimeter or heat transferred to the environment, and the heat lost by one substance equals the heat gained by the other.
Heat provided to or released from a calorimeter can be calculated using specific equations that incorporate variables such as:
- electric current
- voltage
- time
- calorimeter constant
- temperature change
This allows us to understand the intricacies of heat flow and how it affects chemical and physical processes.
Types of Calorimeters
Similar to the wide-ranging applications of calorimetry, the devices employed to measure heat changes are also diverse. They are classified based on their operational methods, which include isothermal, adiabatic, heat-flow, and isoperibol calorimeters, among others. Calorimeters range in design from simple coffee-cup calorimeters with Styrofoam insulation to industrial reaction calorimeters like heat transfer fluid flow, power compensation, and constant flux calorimeters.
Certain advanced designs incorporate vacuum jackets to minimize heat loss, while some types encounter limitations with certain reactions, such as slow or highly energetic ones, under constant pressure. For instance, Calvet-type calorimeters utilize three-dimensional fluxmeter sensors for detection, providing absolute calibration of maximum temperature and the ability to increase the experimental vessel size without affecting accuracy.
Bomb Calorimeters
Bomb calorimeters are commonly used to measure heat in reactions. These advanced devices operate at constant volumes. They are ideal for measuring the heat of combustion. A typical bomb calorimeter is a complex setup consisting of:
- A sample cup
- Oxygen
- A stainless steel bomb
- Water
- A stirrer
- A thermometer
- Insulating materials
- An ignition circuit
A coffee cup calorimeter is a simpler device for measuring heat changes in solution reactions, making it popular for education. During bomb calorimetry, measure the temperature increase in surrounding water from the initial temperature before igniting the sample in the constant-volume container. Then calculate the energy produced by the reaction using this temperature increase. Therefore, bomb calorimeters provide a precise measure of the heat released during combustion.
Adiabatic Calorimeters
Adiabatic calorimeters present a distinctive method for measuring latent heat changes. They are notably used to examine:
- runaway reactions, helping to prevent accidents in industrial processes
- standard entropy
- phase transitions
- thermodynamic functions
These devices are pivotal in theoretical research and the development of new materials.
Automated adiabatic calorimeters leverage modern computer technology to streamline experiments and achieve high-precision measurements of heat capacity and thermodynamic data. Moreover, they use the phi-factor, a ratio that corrects calorimetric results to account for heat losses, enhancing the accuracy of measurements.
Differential Scanning Calorimeters
Differential scanning calorimetry (DSC) operates by heating the sample and reference material separately and adjusting the heat to maintain equal temperatures. This required balance of the heat capacities is directly measured and correlates with the heat evolved or absorbed during the sample’s transformation. Advanced DSC techniques like Fast-scan DSC (FSC) enable high scanning rates to assess rapid phase transitions, while Temperature Modulated DSC (TMDSC) helps separate overlapping effects to analyze time-dependent phenomena and specific heat capacity changes. Thus, the versatility of DSC makes it a valuable tool in various scientific domains.
Through DSC, transition temperatures and the enthalpies of transitions are determined by integrating the area within the DSC peak. This is crucial for constructing phase diagrams. In polymer characterization, DSC detects phase transitions and studies polymer curing. In biochemistry, it offers insights into protein folding and stability through the study of thermal denaturation.
Calorimetry in Action: Practical Applications
Beyond the realm of theoretical science, calorimetry has a plethora of practical applications. From chemical reactions and thermochemistry to biochemistry and pharmaceutical research, even extending to energy and environmental sciences, calorimetry is a cornerstone of modern scientific applications and what does a calorimeter measure.
A significant application of calorimetry is in the chemical industry where Continuous Reaction Calorimeters are used to gather thermodynamic data. This data is crucial for scaling up continuous processes in chemical reactors. The system of a Continuous Reaction Calorimeter includes components such as:
- a tubular reactor
- dosing systems
- preheaters
- temperature sensors
- flow meters
The specific heat of reaction is determined by utilizing heat balances and segmental dynamic parameters. This information is vital for process safety and optimization in the chemical industry.
Chemical Reactions and Thermochemistry
Unraveling the mysteries of chemical reactions and thermochemistry wouldn’t be possible without the aid of calorimetry. Reaction calorimetry has become an essential tool for chemists and chemical engineers to determine reaction kinetics and chemistry, especially for process optimization and safety.
Through the simulation of failure scenarios in a controlled environment, reaction calorimetry provides indispensable insights for the development of safe industrial processes and emergency protocols. Chemical processes are often developed and refined with the assistance of reaction calorimetry. It facilitates the selection and optimization of synthetic routes and thermal profiling.
A full reaction heat calorimeter initiates chemical reactions within a closed insulated container to measure the heat of reaction. There are various types of reaction calorimeters such as:
- Heat flow calorimeters (HFC)
- Power compensated calorimeters (PCC)
- Heat balance calorimeters
- True heat flow calorimeters
These techniques are used to accurately measure the heat generated or heat absorbed during a chemical reaction and in discovering what does a calorimeter measure.
Biochemistry and Pharmaceutical Research
Isothermal titration calorimetry (ITC) assumes a pivotal role in the field of biochemistry and pharmaceutical research. It is instrumental in determining substrate binding to enzymes and investigating ligand-protein interactions, which are foundational to biochemical research.
ITC provides quantitative data by measuring the heat of a reaction, which corresponds to essential thermodynamic parameters such as:
- Enthalpy
- Gibbs free energy
- Reaction midpoints
- Entropy
- Binding affinities
This data aids in the determination of these parameters.
The technique of ITC is gaining vital importance in the pharmaceutical industry, particularly for the purpose of drug development, due to its ability to accurately characterize molecular interactions.
Energy and Environmental Sciences
In the energy and environmental sciences, calorimetry is an essential tool. Continuous Reaction Calorimeters are used to gather thermodynamic data for scaling up continuous processes in chemical reactors. This information is crucial for the development and optimization of energy-efficient and environmentally friendly processes.
The system of a Continuous Reaction Calorimeter includes the following components:
- Tubular reactor
- Dosing systems
- Preheaters
- Temperature sensors
- Flow meters
These elements work together to measure and record the heat changes associated with various chemical reactions.
The specific heat of reaction is determined in Continuous Reaction Calorimeters by utilizing heat balances and segmental dynamic parameters. This information is crucial for process safety and optimization in the energy sector.
Calorimetry Challenges and Limitations
Despite an abundance of applications and benefits, calorimetry comes with its own set of challenges. These include heat loss, calibration, and sensor limitations, which can all potentially lead to inaccurate measurements and errors.
Heat loss to the environment is a significant challenge in calorimetry as it can lead to inaccurate heat measurements. This is particularly a concern in bomb calorimetry, where the higher pressure and concentration of O2 can make non-flammable compounds flammable, leading to harder calculations and potential errors.
Regular calibration of calorimeters is essential to obtain accurate measurements. However, calibration can be a complex process, requiring the use of reference materials and following standard procedures.
Sensor limitations also pose challenges, with contamination from substances like dust, smoke, and oil vapor leading to significant measurement errors.
Heat Loss and Thermal Equilibrium
Calorimetry faces a major challenge in the form of heat loss to the environment. This loss can result in inaccurate heat measurements and consequently, can influence the outcomes of experiments or processes. To mitigate this, insulating the calorimeter with lagging material can help prevent unwanted heat exchange with the environment.
Maintaining an even temperature distribution within the calorimeter is also important. Stirring the liquid inside the calorimeter can help achieve this same temperature, reducing measurement errors. Moreover, placing a lid or cover on the calorimeter can decrease heat loss through evaporation and convection.
In heat balance calorimetry, maintaining accurate measurements requires managing heat loss. This can be achieved by:
- Monitoring the differential coolant temperatures entering and leaving the calorimeter jacket
- Ensuring proper insulation of the calorimeter to minimize heat loss
- Using a constant temperature bath or jacket to maintain thermal equilibrium
By managing heat loss and achieving thermal equilibrium, accurate calorimetry measurements of thermal properties can be obtained.
Calibration and Standardization
The calibration of calorimeters is a vital step towards guaranteeing precise measurements. This procedure entails the use of reference materials and adherence to standard procedures. For instance, heat flow calorimeters require calibration to determine their overall heat transfer coefficient. Calvet-type calorimeters, on the other hand, use a Joule effect or electrical calibration method.
A typical calibration method involves passing a known quantity of electrical current through the calorimeter for a known duration and measuring the resultant temperature change. This process, while essential, can be complex and time-consuming.
Moreover, regular maintenance and cleaning of the calorimetric sensors are crucial to ensure accurate and reliable measurements. The presence of contaminants can alter the sensor’s behavior, leading to potential errors. Thus, calibration and standardization are fundamental to achieving accurate calorimetry results.
Sensor Limitations and Potential Errors
Calorimetry can be significantly hindered by sensor limitations. For instance, calorimetric flow sensors might yield considerable measurement errors when contaminated with substances such as dust, smoke, and oil vapor. Even a thin contamination layer of only 15 μm can lead to measurement errors as high as 25%.
When contaminants are deposited on the upstream area of the sensor chip, they can particularly alter sensor readings, resulting in incorrect calorimetric data. Moreover, both calorimeter-type and thermocouple-type sensors suffer from short exposure duration, low thermal conductivity, and challenges in discerning the sensor’s temperature from that of the fabric.
Optical sensors also encounter difficulties in assessing a significant portion of the transmitted heat and the conductive and convective heat flux components. Thus, understanding sensor limitations and potential errors is essential for accurate calorimetry.
Summary
What does a calorimeter measure? Calorimetry, with its intricate science and multitude of applications, continues to be an indispensable tool in the world of science. From exploring the fundamentals of heat capacity and specific heat to understanding the intricacies of various types of calorimeters, we have embarked on an enlightening journey. Despite its challenges, including heat loss, calibration, and sensor limitations, the field of calorimetry remains pivotal in chemical reactions, biochemistry, pharmaceutical research, and energy and environmental sciences. As we continue to refine our techniques and overcome limitations, the future of calorimetry shines bright with promise.
Frequently Asked Questions
Does calorimeter measure temperature change? What does a calorimeter measure?
Yes, a calorimeter measures temperature change to determine the amount of thermal and electrical energy input transferred in a process. This is what does a calorimeter measure.
What is calorimetric measurement?
Calorimetry is a fundamental method to measure the absorbed energy in matter due to radiation. It involves measuring the increase in temperature and comparing it with a calibrated heat source in the process of discovering what does a calorimeter measure.
Can a calorimeter measure energy?
Yes, a calorimeter can measure energy by carefully measuring the temperature change in a chemical or physical process and the masses of the system and surroundings.
What are some types of calorimeters?
There are several types of calorimeters, including bomb calorimeters, adiabatic calorimeters, and differential scanning calorimeters, each with its own distinct operational methods and designs.
what does a calorimeter measure
How is heat measured in a bomb calorimeter?
Observing the temperature increase in the surrounding water after igniting the sample in the constant-volume container measures heat in a bomb calorimeter. This temperature change is then used to calculate the energy or heat produced by the reaction.
Related Blogs for What Does a Calorimeter Measure:
Understanding Air Condenser Chemistry in 2023
Chemistry Glassware Names: A Comprehensive Guide
Suction Filtration: The Basics of Vacuum Filtration
What is a Graduated Cylinder Used For?
Most Accurate Glassware for Measuring Volume
All About Volumetric Flask: Uses, Function & Overview
Aspirator Flask: Benefits of Borosilicate Glass Filter Flask
Comprehensive List of Biology Laboratory Equipment in 2023
Everything You Need to Know About Beakers in Chemistry
Pipette or Measuring Cylinder: Tools for Liquid Measurements
Measuring the Volume of Liquid: Tips, Tools, and Techniques
Unlock the Secrets of Flask Volume for Accurate Results
Efficiency with the Erlenmeyer Flask: Tips for Lab Use
Guide to Bunsen Burners: Types, Uses, and Safety Tips
Lab Burner: Safe and Efficient Heating in the Laboratory
Calculate Heat Index: Understanding the Feel of Temperature
What is the Boiling Point of Water in Celsius: A Clear Guide