Dealing with a mercury thermometer requires careful attention to both its accuracy and associated hazards. This article serves as a practical guide, directly addressing the use, calibration, safe disposal, and regulatory aspects, as well as the environmental concerns of mercury thermometers, without overlooking the shift towards safer alternatives.
Key Takeaways
- Mercury thermometers offer precision due to mercury’s unique properties, require regular maintenance for accuracy, and have alternatives like digital and bimetal thermometers for a range of temperatures.
- Mercury has severe environmental and health risks, including neurological damage and developmental deficits; broken thermometers must be handled with extreme care to avoid mercury exposure.
- Due to the dangers of mercury, proper disposal of mercury thermometers is crucial, and there are regulatory efforts to reduce their use in favor of non-mercury alternatives.
Basics

Mercury thermometers have a rich history, tracing back to the early 18th century, with significant contributions from Fahrenheit, leading to their widespread adoption over devices sensitive to atmospheric pressure variations. These thermometers are constructed of a tubular glass, with a mercury-filled reservoir at the tube’ base and a capillary bore through the stem, making them efficient instruments to measure temperature.
The accuracy and ease of reading mercury thermometers are due to the following factors:
- Quick response of mercury to temperature changes
- High level of conductivity
- Uniform thermal expansion
- Non-adherence to glass
- Visibility
These thermometers have an operational temperature range typically from 35 to 673 degrees Fahrenheit, which can be extended to higher temperatures with inert gas and specialized materials. The principle is simple: mercury moves through the bore as it expands or contracts in accordance with temperature changes.
However, like all tools, mercury thermometers are not immune to wear and tear. Their accuracy can degrade over time due to factors such as:
- Calibration
- Handling
- Environmental conditions
- Natural wear on the capillary and mercury column
Regular maintenance and calibration can help in mitigating these challenges and ensuring accurate battery readings.
Measuring Body Temperature
Mercury thermometers are not just limited to meteorological or laboratory use; they have been a reliable tool for measuring body temperature. This can be done orally, rectally, or axillary (armpit). Each method has its own procedure and results in varying temperature readings.
An oral temperature reading involves placing the mercury thermometer under the tongue, closing the mouth, and waiting for about five minutes. For measuring armpit temperature, the thermometer is placed in the deepest crease of the armpit and held in place for approximately five minutes with the arm closed. For newborns, the most accurate measurement is the rectal temperature, which involves lubricating the thermometer’s tip, gently inserting it rectally, and waiting for about five minutes.
Once the body temperature is measured, it’s imperative to thoroughly read the mercury column on the thermometer. Make sure to consider that temperature readings can differ based on the location of measurement:
- Oral temperatures typically average 98.6 degrees Fahrenheit
- Rectal temperatures are about 0.5 to 1.0 degrees higher
- Armpit temperatures usually turn out to be 0.5 to 1.0 degrees lower.
Precautions
Despite the efficiency of mercury thermometers in measuring body temperature, certain precautions must be taken to guarantee both accuracy and safety. Specifically, they should not be used for children under age 4 who cannot keep their mouths closed long enough to get a good reading. It is also recommended to wait 20 to 30 minutes after eating or drinking before taking an oral temperature with a mercury thermometer.
When taking a rectal temperature with a mercury thermometer, follow these steps:
- Insert the thermometer only about a half inch, and do not force it if resistance is felt.
- Cleaning or disinfecting the thermometer before and after use is vital to prevent the spread of germs.
- Above all, handle the thermometer with care. Avoid shaking or dropping it, as mishandling can lead to inaccuracies in temperature readings.
Mercury-Free Alternatives
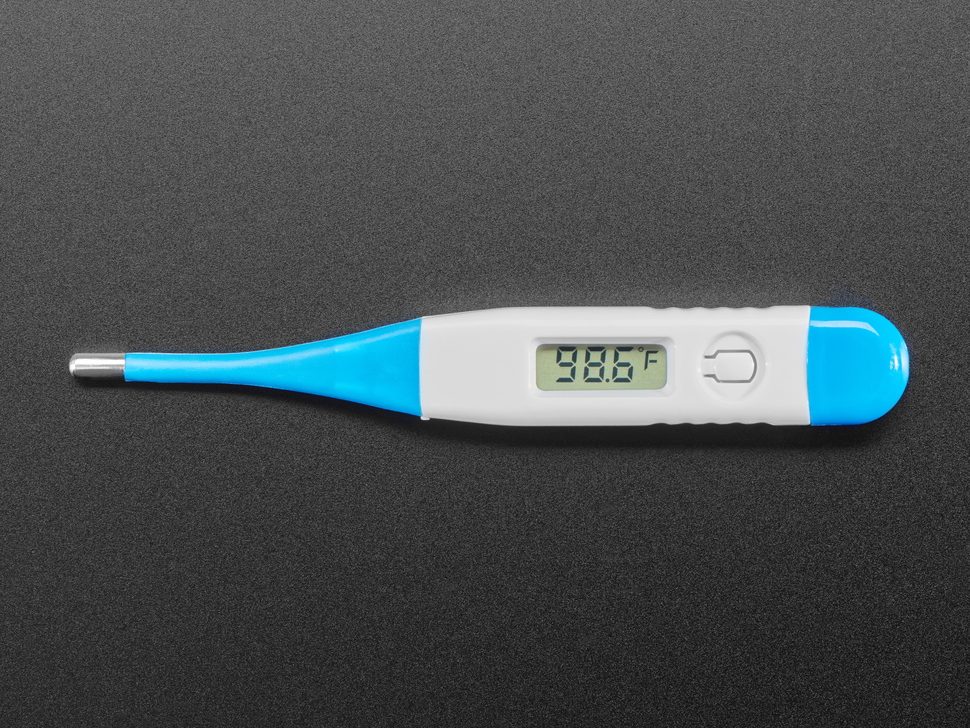
While mercury thermometers have been a staple for centuries, advancements in technology have brought forward mercury-free alternatives. Digital thermometers, such as those utilizing sensors like platinum resistance (PRT), thermistors, and thermocouples, offer an alternative to mercury containing thermometers. Bi metal thermometers, another mercury-free option, are capable of measuring temperatures ranging from -200 °C to above 500 °C, depending on the sensor type.
Another practical alternative is the glass thermometer, specifically non-mercury liquid-in-glass thermometers. These provide accurate measurements with nominal uncertainties as low as 0.02 °C, catering to temperature ranges from -196 °C to 300 °C. The selection of electronic thermometers, such as digital thermometer sensors, is dependent on the desired level of measurement accuracy, application environment, and potential exposure to mechanical shock. These thermometers can also offer advanced features, such as minimum and maximum temperature display, alarms, and options for connectivity, such as wireless or USB. However, certain conditions or damage may render a thermometer unusable, making it crucial to choose the right type for your specific needs.
Globally, there’s a growing trend of transitioning towards mercury-free thermometers in medicine. Countries like the Philippines and Argentina have mandated the phase-out of mercury-in-glass thermometers from hospitals, opting for non-mercury digital thermometers.
Environmental and Health Concerns

The shift towards mercury-free alternatives is not just driven by technological advancements, but also by the environmental and health concerns associated with mercury. Mercury exposure can lead to severe health risks, including neurological problems and developmental deficits in children. Methylmercury exposure, often through the consumption of contaminated seafood, is recognized as a powerful neurotoxin. It can impair vision, coordination, speech, and muscle strength. Ingesting methylmercury during pregnancy can lead to developmental deficits in children, affecting cognitive thinking, memory, attention, language, fine motor skills, and visual spatial skills.
Inhalation of metallic mercury vapor, common when mercury-containing products break, can result in tremors, emotional instability, insomnia, neuromuscular changes, headaches, sensory disorders, and altered nerve responses. Excessive exposure to mercury can cause detrimental health effects including mood swings, memory loss, kidney and gastrointestinal damage, and at high levels can cause respiratory failure and death. Brief contact with high concentrations of mercury can trigger immediate health effects, such as loss of appetite, fatigue, insomnia, and changes in behavior or personality.
Besides health risks, mercury also poses significant environmental threats. Environmental release of mercury from broken thermometers contributes to the production of methylmercury, a highly toxic compound that becomes concentrated in seafood, posing significant health threats. As mercury solidifies, it becomes even more challenging to clean up, further exacerbating the issue.
Handling Broken Thermometers with Mercury

Due to the health and environmental risks posed by mercury, handling broken mercury thermometers demands utmost care. If you break a mercury thermometer, follow these steps:
- Immediately try to contain the spill.
- Keep children and pets away.
- Avoid using a vacuum cleaner or broom to prevent vaporization and the spread of mercury.
While handling broken glass, wear gloves and be cognizant that contaminated clothing should not be worn to prevent spreading the mercury. Use items like gloves, eye droppers, plastic bags, cardboard or squeegees, and adhesive tape to collect mercury beads and safely manage a cleanup. Optional powdered sulfur can be used to detect and absorb mercury, and a flashlight can help spot any remaining small beads.
Following the cleanup, ensure the area is ventilated for at least 24 hours to allow any mercury vapors to dissipate. For mercury vapor monitoring or if the spill is extensive, consult a contractor with the right equipment and secure the cleanup materials for disposal by a licensed and certified hazardous waste contractor.
Proper Disposal

To avoid environmental contamination, mercury thermometers must be disposed of properly. Improper disposal introduces mercury vapor to the air and liquid mercury to landfills. It’s crucial to follow local regulations for mercury waste disposal. States and local jurisdictions may have unique regulations, and residents can access collection and exchange programs through local officials.
Mercury thermometers awaiting disposal should be stored in an airtight, leak-proof container labeled as containing mercury, and secured in a cardboard box during transport to a hazardous waste facility. To prevent environmental contamination, mercury should not be poured down drains, and containers with mercury thermometers should be labeled ‘DO NOT OPEN’ and kept out of reach of children and pets.
Regulations and Restrictions
Given the health and environmental risks posed by mercury, numerous organizations are striving to limit the use of mercury thermometers while advocating for mercury-free alternatives. for example, The EPA has collaborated with various stakeholders to diminish the usage of mercury-containing non-fever thermometers in industrial and commercial sectors across the United States.
In January 2012, the EPA’s rule revision facilitated alternative options to mercury thermometers in regulations concerning petroleum refining, electricity production and distribution, and polychlorinated biphenyl (PCB) waste management. Through partnerships with ASTM International and the American Petroleum Institute (API), the EPA has fostered the inclusion of non-mercury alternatives within industry standards, ensuring regulatory flexibility.
On a similar note, the National Institute of Standards and Technology (NIST) ceased the calibration service for mercury-in-glass thermometers on March 1, 2011, to promote traceability, supporting the move away from mercury thermometer utilization. Despite these regulatory updates, some U.S. federal and state regulations still necessitate the use of mercury thermometers or reference specifications for their use through standards by ASTM International and API.
Calibration and Maintenance
To ensure optimal performance of mercury thermometers, both accurate calibration and consistent maintenance are crucial. Calibration involves bringing the thermometer to thermal equilibrium with fixed points such as ice water, where it should read 0 degrees Celsius, and boiling water, where it should read 100 degrees Celsius at sea level. Keep in mind that atmospheric conditions and altitude may alter the boiling point of water, necessitating local adjustments to calibrate the calibration process.
Some mercury thermometers have a calibration nut that allows for fine adjustments while immersed in the reference temperature. When calibrated accurately, mercury thermometers typically achieve a reading accuracy within ±0.1 to ±0.2 degrees Celsius. Regular accuracy checks at known temperature points are crucial, even when recalibration is not possible.
In some cases, the upper temperature limit for mercury thermometers can be increased by adding an inert gas above the mercury or using fused quartz instead of mercury in glass thermometer. However, these measures should be taken with utmost caution due to the hazardous nature of mercury, especially when dealing with lower meteorological temperatures.
Summary
Understanding mercury thermometers is a crucial aspect of safe and effective use. From their construction to calibration, these devices require careful handling and maintenance to ensure accurate temperature readings. While mercury thermometers have been widely used for centuries, the health and environmental concerns associated with mercury have led to the development, testing and adoption of mercury-free alternatives.
It’s important to remember that when dealing with mercury thermometers, safety should always be the top priority. From handling broken thermometers to proper disposal, each step should be taken with caution to prevent potential health risks and environmental contamination. As we continue to innovate and develop safer, more sustainable alternatives, let’s also do our part by being mindful users and responsible custodians of our environment.
Certified MTP has numerous options for the thermometers, including Mercury in Glass Thermometers, ASTM Mercury Filled Thermometers, ASTM Non-Mercury Thermometers, Mercury Free Thermometers, and Digital Infrared Thermometers.
Frequently Asked Questions
Do they still make thermometers with mercury?
These thermometers are mostly being phased out due to laws and regulations, and are being replaced by digital or non-mercury options. However, they are still commonly used in certain industries.
Are thermometers with mercury still accurate?
These thermometers are accurate, but they come with drawbacks such as toxicity, fragility, and a high freezing point. There is no significant difference in accuracy compared to electronic thermometers, but electronic thermometers have a greater fluctuation of readings.
What is a thermometer with mercury used for?
These thermometers were traditionally used for measuring outdoor temperatures and body temperatures, but due to the highly hazardous properties of mercury, such as its extreme toxicity and the risk of organ and nerve damage if the glass is damaged, they are no longer recommended for use.
Why were thermometers with mercury banned?
These thermometers were banned due to the health and environmental hazards of mercury release if they break, and many countries have banned them for medical use due to the toxicity of mercury. There is a risk of dangerous exposures to mercury vapor in indoor air when these thermometers break.
How do thermometers with mercury work?
These thermometers work by utilizing the thermal expansion and contraction of liquid mercury, which moves through a capillary bore to indicate the temperature based on the expansion of flow or contraction of the mercury.
Related Blogs for Mercury Thermometers:
Precision ASTM Thermometers: Certification and Specification
Thermometers with Mercury: Everything You Need to Know
How to Convert 65 Degrees C to F>
Role of Thermometers in Concrete Curing
How to Convert 70 Deg F to C Easily
How to Convert 158 Fahrenheit to Celsius (158 F to C)
Lab Grade Thermometer for Concrete Testing
Certified Thermometers for Material Testing
Comprehensive List of Biology Laboratory Equipment in 2023
Thermometers Calibrated: Calibrate for Accurate Readings
Convert 900 Degrees Fahrenheit to Celsius (900 F to C)
Master C to F Formula: How to Convert Celsius to Fahrenheit
Digital Humidity and Temperature Meter: Discover the Best
7 Fahrenheit to Celsius: Quick Temperature Conversion Guide
75 Fahrenheit to Celsius Converter: Easy Conversion Method
Easy Guide: Convert 69 Fahrenheit to Celsius Effortlessly
67 F to C: Accurate Fahrenheit to Celsius Conversion Guide
Convert 99.4 F to C: Fahrenheit to Celsius Conversion
Easy K to F Conversion: Your Guide to Kelvin to Fahrenheit
Quick 35 f to c Conversion: Convert Fahrenheit to Celsius
Easy 0 F to C Conversion: Turn Fahrenheit into Celsius Fast
60 Degrees C to F: Quick Conversion Guide
32 Celsius to Fahrenheit: Easy Temperature Conversion Guide
How to Convert 79 Degrees F to C Easily [Conversion Solved]
Temperature Conversions Table: Fahrenheit to Celsius
Celsius to Kelvin: Guide to Accurate Temperature Conversion
Quick and Easy Celsius Calculator for Temperature Conversion
Fahrenheit to Rankine Formula: Steps for Temp Conversion
Rankine to Celsius Conversion: Your Complete R to C Guide
Is Mercury Illegal to Own? Understanding Regulations
Degrees in Radians: A Guide for Converting Angles
Discover the Best Type of Thermometers for Accurate Readings
Accurate and Reliable Glass Thermometer Selection Guide
Mercury Thermometers: Safe Disposal Guidelines
78F C Conversion Guide: Easily Convert Fahrenheit to Celsius


1 Comment
Каталог финансовых продуктов: ваш гид в мире финансов
Ищете удобный способ сравнить и выбрать финансовые продукты? Загляните в наш каталог финансовых продуктов! У нас есть все, что вам нужно, от кредитов и дебетовых карт до сберегательных счетов и инвестиций.
кредит взять
С нашим удобным поисковым инструментом вы можете быстро и легко найти продукты, соответствующие вашим конкретным потребностям. А благодаря нашим подробным обзорам и сравнениям вы можете быть уверены, что принимаете обоснованное решение.